April 30
@
4:00 PM
–
5:00 PM
Curtis Berlinguette
Department of Chemistry and Chemical and Biomolecular Engineering
University of British Columbia
Vancouver, BC
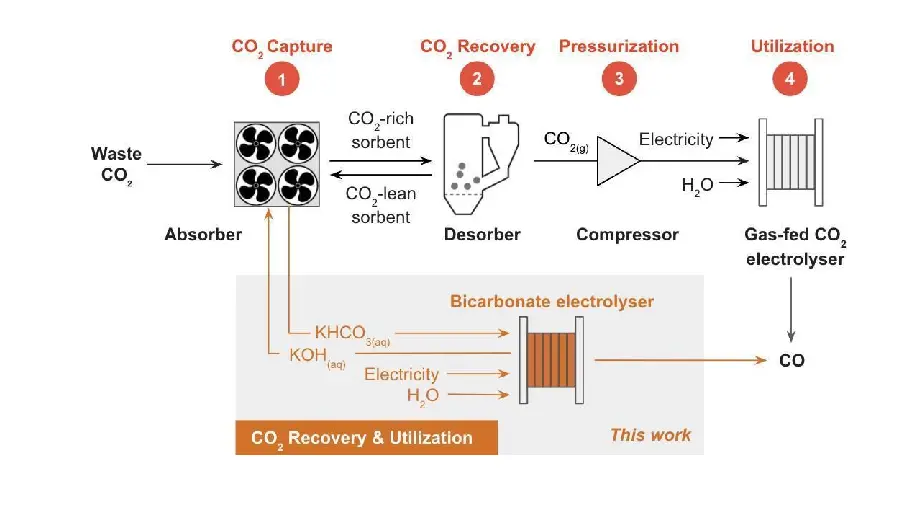
Reactive Carbon Capture
Carbon capture and utilization schemes require that CO2 captured from the atmosphere (or a point source) be released from the sorbent, and that the sorbent be recycled to capture additional CO2. Alkaline solutions such as KOH are effective at capturing CO2 through reactions that form (bi)carbonates, but the recovery of CO2 gas and hydroxide before CO2 electrolysis requires energy-intensive steps. We solved this problem by designing an electrochemical reactor that converts bicarbonate “reactive carbon capture solutions” into carbon-containing products. In this presentation, I will show how this reactor couples CO2 conversion with upstream carbon capture. Not only does this reactor bypass the expensive step of liberating CO2 from the sorbent, but it can also perform better than the reactors fed with gaseous CO2.