One way to treat the most common type of kidney cancer is to use anti-angiogenic drugs to cut off the blood supply to the tumors, but patients respond differently to the drugs, and choosing the wrong one can make the cancer grow faster.
A new study by researchers from the University of Wisconsin Carbone Cancer Center and the Department of Biomedical Engineering, published online this week in EBioMedicine, has developed a model that mimics the tumor’s blood supply on a three-dimensional platform designed in the laboratory of John D. MacArthur Professor and Claude Bernard Professor David Beebe.
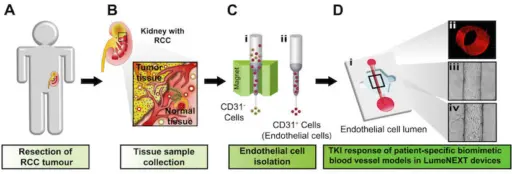 Tumor and normal tissue are removed from the patient during surgery, the cells are isolated, and then injected into the lumen device created by the Beebe lab. There they grew into three-dimensional models of kidney cancer blood vessels.
Tumor and normal tissue are removed from the patient during surgery, the cells are isolated, and then injected into the lumen device created by the Beebe lab. There they grew into three-dimensional models of kidney cancer blood vessels.
The authors of the study, led by Beebe lab members Jose “Tony” Jimenez-Torres and Maria Virumbrales-Munoz, used both normal and cancerous tissue from patients to grow the blood vessels. They replicated many of the structures seen in the normal and cancer-tissue blood supplies and used them to test targeted drugs used to treat renal cell carcinoma.
Renal cell carcinoma is the third-most common genitourinary cancer in the United States, with about 64,000 new cases each year. This kidney cancer tends not to respond to standard chemotherapy, so anti-angiogenic targeted agents, which can cut off the blood supply to tumors by inhibiting vessel formation, are often used.
“In this type of cancer, clinicians report that giving the patients the wrong drug can be counterproductive,” says Virumbrales-Munoz, a postdoctoral fellow. “You can actually increase angiogenesis and feed the tumor more. This screening approach could help us make treatment decisions for each patient, knowing beforehand the outcome of their treatment.”
Jimenez-Torres, manager of microtechnology core in Beebe’s research group, says the lab was able to create an in vitro model of each patient’s tumor blood vessels to test the effect of anti-angiogenic drugs. The normalization of tumor blood vessels in response to anti-angiogenic drugs would lead to decreases in tumor size.
“This is the first model for renal cell carcinoma that enables personalized anti-angiogenic drug testing’’ explains Jimenez-Torres.
The preliminary data of this study was so promising that the group was awarded a $1.5 million Cancer Moonshot grant from the National Cancer Institute to compare the results of the personalized model with patient response.
The “moonshot” clinical study will use positron emission tomography (PET) imaging before and after the patients are treated for their cancer, to see whether it responded to a particular drug. Meanwhile, in the Beebe lab, the tumor tissue will be grown into three-dimensional blood vessel structures and treated with the drugs. The goal is to see whether the in vitro model predicts the patient response.
“Our future goal is not to test every single patient, but to develop a list of markers that will better predict which patient will respond to a treatment,’’ says Virumbrales-Munoz.
Other collaborators on the project include: Jason Abel, associate professor of urology; Christos Kyriakopoulos, assistant professor of hematology-oncology; Wei Huang, professor of pathology; Steve Y. Cho, associate professor of radiology; and KyungMann Kim, professor of biostatistics.