Tom Turng envisions a future in which surgeons can order mass-produced artificial blood vessels that arrive ready to use in bypass surgeries.
“Kind of like when you order something off Amazon and it ships right away; we want to do the same thing—but instead, the off-the-shelf product is artificial blood vessels that doctors can implant into a patient,” says Turng, Kuo K. and Cindy F. Wang Professor and Vilas Distinguished Achievement Professor in mechanical engineering at the University of Wisconsin-Madison.
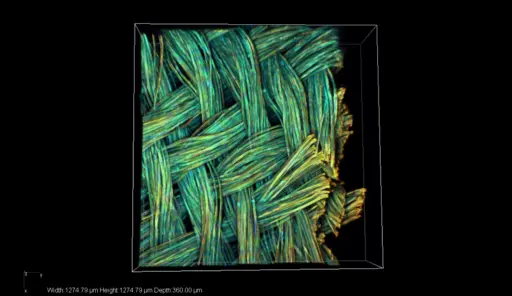 The first generation of the researchers’ artificial blood vessel used braided silk fibroin. Submitted photo.
The first generation of the researchers’ artificial blood vessel used braided silk fibroin. Submitted photo.
A pioneer in bio-based materials for use in the body, Turng has developed a way to consistently create small-diameter artificial vascular grafts that mimic the mechanical performance of native blood vessels. He and his collaborators reported their advance in the Jan. 28, 2019, edition of the Journal of Biomedical Materials Research: Part B – Applied Biomaterials.
Currently, artificial blood vessels with diameters smaller than 6 millimeters—the kind needed for bypass surgeries—are not commercially available. However, such a product could transform treatment for cardiovascular diseases, which are the leading cause of death globally.
Turng’s invention, which promises to eliminate the need to harvest vessels from the patient, addresses this major medical need. Each year, for example, more than 500,000 bypass surgeries are performed in the United States alone.
Today, surgeons performing bypass procedures must remove veins or arteries from another part of the patient’s body. Then, they implant those vessels to create a new route for blood flow that bypasses a blocked or diseased vessel.
“However, suitable tissue isn’t often available due to prior harvesting or a patient’s health, so in those cases we need to have an artificial blood vessel to complete the bypass,” Turng says.
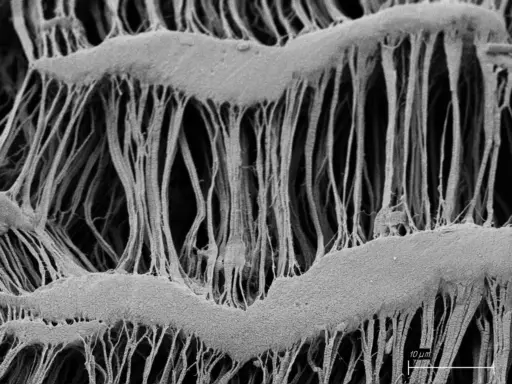 A SEM image of the microstructure of an expanded artificial blood vessel. The wavy structures of the second-generation vessel allow it to mimic the mechanical performance of native blood vessels. Image courtesy of Tom Turng.
A SEM image of the microstructure of an expanded artificial blood vessel. The wavy structures of the second-generation vessel allow it to mimic the mechanical performance of native blood vessels. Image courtesy of Tom Turng.
But not just any artificial blood vessel will do.
Turng says it’s crucial for these artificial blood vessels to emulate the mechanical properties and performance of natural blood vessels. “In the small-diameter blood vessels that we’re working with, even a slight mismatch in mechanical properties will lead to the formations of blood clots and other complications that can be deadly,” he says.
When we engage in physical activity, our blood vessels can easily expand to allow extra blood flow and oxygen to sustain that activity. But when a vessel reaches a certain expansion, it also stiffens to maintain its integrity for blood circulation. This type of behavior—initially soft and subsequently stiff—is challenging to replicate with artificial blood vessels.
Turng and his collaborators drew on their extensive experience in polymer processing and materials selection in their quest to find the optimal combination of materials and fabrication methods—including braiding, electro-spinning, thermal-induce phase separation and extrusion—to produce an artificial vessel with lifelike mechanical properties.
In the end, their winning combination included using a stretchy plastic material along with stiffer biomaterials that emulate the elastin and collagen fibers in a blood vessel, respectively. Turng found that creating wavy structures with the stronger biomaterials caused the vessel to toughen once it reached full expansion, mimicking the behavior of the real thing.
This ambitious ongoing research project involves close collaboration with stem-cell pioneer James Thomson, director of regenerative biology at the Morgridge Institute for Research at UW-Madison. Thomson’s research group is using induced pluripotent stem cells to engineer the endothelial cells that make up the cellular lining of the vessel. The collaboration has enabled the team to narrow down viable materials.
Meanwhile, Turng has also developed sophisticated methods for modifying the surface of the vessels. These modifications allow the vessel to function as the scaffolding upon which the endothelial cells will adhere and grow outside of a patient’s body.
“Once the endothelial cells fully cover the interior of the artificial blood vessels and form a congruent state, the blood vessel is ready to be used,” Turng says. “Those cells will die and new ones will regenerate, as do normal blood vessels.”
By using stem cells from a unique population of people who are genetically compatible donors, the researchers hope to create artificial blood vessels that won’t be rejected by a patient’s body following a transplant. Cells from these “universal donors” would enable researchers to mass produce artificial blood vessels as off-the-shelf products that are biologically compatible with the majority of patients who need bypass surgery.
“Right now, we’re conducting cell culture experiments, and we’re striving toward the critical phase of proving that we can grow the endothelial cells inside the fabricated tube,” says Turng. “Once that’s accomplished, I think we’re pretty close to achieving our mission.”
It’s a mission that Turng says he’s been working on from “day one” since moving his lab into the Wisconsin Institute for Discovery at UW-Madison in 2010. “After all these years of work, I can finally see the light at the end of the tunnel,” he says. “It’s all possible because of the great environment the Wisconsin Institute for Discovery provides and the ability to collaborate with James Thomson’s group and other collaborators to address this pressing challenge.”
The researchers have filed a patent for their technology through the Wisconsin Alumni Research Foundation.
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI). (Grant Number: U01HL134655).