The human body’s circulatory system pumps oxygen and glucose to trillions of cells, providing them with essential energy and nutrients.
Inspired by the body’s example, a team led by James Pikul, an associate professor of mechanical engineering at the University of Wisconsin-Madison, has created a liquid energy storage and delivery system that could power future robots and make chemical manufacturing more sustainable. The researchers detail their work in a paper published September 10, 2024, in the journal Matter.
The project is a unique twist on traditional electrochemical systems that use gases, like metal-air batteries or fuel cells, says Pikul. In those systems, the gas, along with a liquid electrolyte and a current collector supplying electrons, must come together for the necessary chemical reaction to occur.
“The current approach to achieve this looks like a scaled-down version of sponge floating on the surface of a lake,” says Pikul, who works at the intersection of energy conversion and storage, robotics and multifunctional materials. “Ions move to and from the surface through the water, where they meet the air and the sponge facilitates the movement of electrons to this interface. The problem with this approach is the reaction gets blocked when the sponge gets too wet—so no air gets inside—or dries up on a hot day, and the maximum speed of the reaction is limited by the total surface area of the lake.”
In their bioinspired solution, Pikul’s team injected gas directly into the electrolyte using an emulsion of silicone oil droplets in water. The silicone oil can store six times more gas than the water alone, making it behave like hemogoblin in blood. In the lake analogy, the air gets pumped into the lake water as bubbles, and now the entire volume of the lake can participate in the reaction.
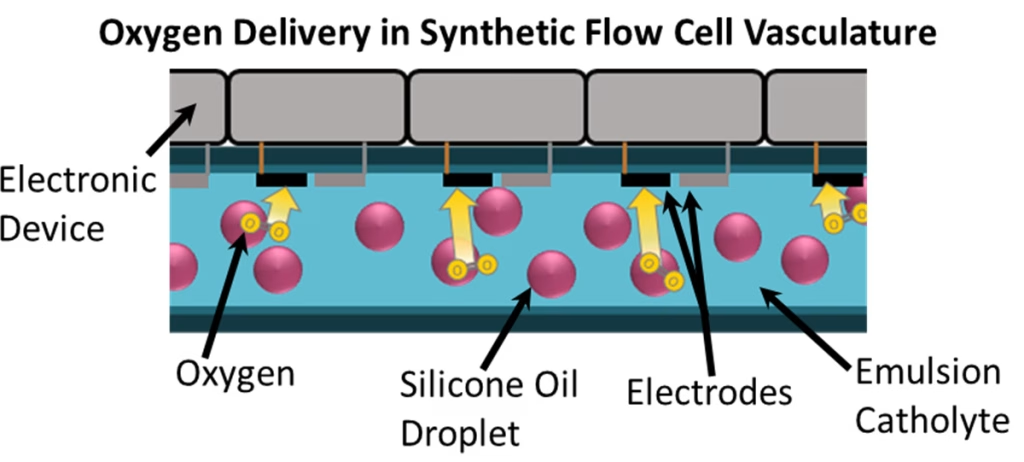
While the group’s current application is to make robots last longer and have more life-like functionality, Pikul says the technology could work in a range of electrochemical systems.
“Gases are used with electrochemistry in many modern-day systems,” says Pikul. “If you want convert carbon dioxide into a fuel using electricity from solar panels, or use electricity to turn carbon dioxide into the building blocks of other chemicals, you need to bring billions of tons of gas into contact with electrolytes. Our approach hopes to make that process cheaper and require less space.”
The work builds off a 2019 Nature paper, in which Pikul and collaborators developed a different synthetic vascular system for a fishlike soft robot that tripled the total time the fish could swim when just powered by lithium-ion batteries. Similar to the current work, the liquid was multifunctional, so that it could store energy and serve as a hydraulic actuator, moving the mechanical components needed for swimming.
This time, though, Pikul and his team wanted to create a liquid that could use oxygen from the air around the robot. They were inspired by older efforts to create synthetic blood and liquid breathing for deep-sea divers. Rather than relying on fluorinated compounds like those prior experiments, study first author Alissa Johnson found silicone oil offered better stability when mixed with water. Ultimately, an emulsion consisting of 20% silicone oil provided the optimal balance of increased oxygen storage and ionic conductivity for the group’s battery system.
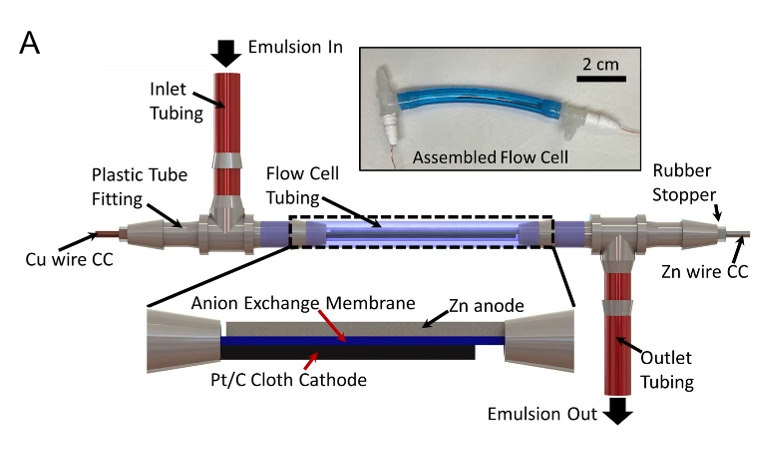
“You can’t have too much oil, because then you won’t be able to conduct ions and your battery won’t work, but if you have too much water, then you’re not really getting the benefits of increasing oxygen solubility,” says Johnson, who conducted the research as a PhD student under Pikul at the University of Pennsylvania. She’s now a postdoctoral researcher at the French National Centre for Scientific Research.
When compared to a conventional electrolyte (the substance that carries electrical current between the anode and cathode in a battery), the researchers’ emulsion produced faster chemical reactions and delivered higher currents.
Moving forward, Pikul is interested in attempting to replace the zinc anodes with a liquid to create a fully liquid power system.
“If we’re going to continue to push the boundaries of science, we have to look at the intersection of different fields that we might not necessarily feel are super-related, and take inspiration from biology,” says Johnson. “We make machines very differently from the way that humans or other animals work, and they’ve had years of evolution to perfect. So why shouldn’t we look at the circulatory system and say, ‘Why can’t we make an energy source that’s based off a continuous feed of materials rather than plugging something in and recharging it?’”
Two multifunctional actuator flow cells
Video caption: In this demonstration video, two of the Pikul lab’s flow cells function as actuators, inflating and deflating, while also powering a connected LED light.
James Pikul, pictured far right in the top photo alongside members of his research lab, is the Leon and Elizabeth Janssen Associate Professor in the Department of Mechanical Engineering. This research was funded by the National Science Foundation (CAREER Award #2344714 and GRFP Award #1845298) and the Office of Naval Research (grant #N000142212569). This work was carried out, in part, at the Singh Center for Nanotechnology at the University of Pennsylvania.