Vascular replacement is a pretty common surgery in the United States, with an average of 450,000 patients per year receiving vascular grafts to treat blood clots, coronary disease, stroke damage and other problems. Once surgeons place a new blood vessel, its health is monitored by CT scans, ultrasounds and other expensive imaging techniques. Despite all that effort, between 40 and 50% of those grafts fail each year, leading to more surgery and, in some cases, death.
 Xudong Wang
Xudong Wang
That’s one reason University of Wisconsin-Madison materials science engineers are developing a new 3D-printed artificial blood vessel that allows doctors and patients to keep tabs on its health remotely.
In a new paper in the July 21, 2020, issue of Advanced Functional Materials, Professor Xudong Wang and lead author Jun Li present a flexible ferroelectric composite that they 3D printed into an artificial artery capable of real-time monitoring.
“This artificial vessel can produce electric pulses based on pressure fluctuation which will be able to tell precisely the blood pressure in the vessel without using any additional power source,” says Wang. “And because of the 3D geometry, the electric pulse profile will be able to tell if there is an irregular motion due to blockage inside in the very early stages.”
The artery project stems from Wang’s long-term research interest in novel soft, flexible biocompatible piezoelectric materials, or substances that can be used in the human body without causing rejection or damage. In order to achieve instantaneous poling during 3D printing, the team combined functionalized sodium potassium niobite piezoceramic nanoparticles with ferroelectric polyvinylidene fluoride polymer.
 Jun Li
Jun Li
They then printed a tubular artery using the material and an off-the-shelf 3D printer integrated with an additional electrical power source and a poling electrode. In their design, the printer extrudes the material through a strong electric field close to the nozzle to polarize the ceramic particles, giving the structure a piezoelectric property, or the ability to produce an electric charge from mechanical stress.
Li put the artificial artery through its paces, hooking it up to an artificial heart system before simulating blockages, high blood pressure and other issues that face artificial blood vessels. The self-powered material was able to correctly detect changes in force and pressure within the artery.
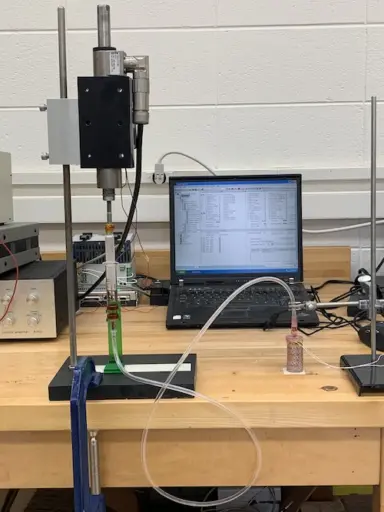 The team tested the self-powered artery using a simulated artificial heart system. Photo: Jun Li.
The team tested the self-powered artery using a simulated artificial heart system. Photo: Jun Li.
The immediate next step is to optimize the production of the novel ferroelectric composite and the 3D printing process. The team also hopes to find ways to make the as-printed 3D structure even more sensitive and plans to collaborate with researchers in the biomedical field to test the artery with even more realistic models of the circulatory system.
Additionally, they hope to use the new material to print artificial heart valves. Currently, replacement heart valves are either mechanical or come from human or animal donors, but none incorporate the type of self-monitoring found in Wang’s material. The team also believes in the future it may be possible to use the ferroelectric biomaterial and 3D printer to create other custom, artificial organs.
While researchers are working on many other types of artificial blood vessels and organs, Wang thinks this technique has some advantages over more complicated techniques.
“This is an easy, scalable technology,” he says. “Our new printable composite material allows us to make a 3D structure in one step that can show multi-functionality right out of manufacture.”
Other UW-Madison authors include Yin Long, Fan Yang, Ziyi Zhang, Yizhan Wang, Jingyu Wang, Corey Carlos and Yutao Dong. UW Molecular Imaging and Nanotechnology Lab director Weibo Cai and visiting assistant professor Hao Wei in the Department of Radiology in the University of Wisconsin School of Medicine and Public Health also contributed to the paper. Cheng Li and Yongjun Wu of Zhejiang University were also involved in the research.
This work was funded by National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB021336. Jun Li is partially supported by a Johnson Controls Graduate Fellowship.