In the wake of a spinal cord injury, a protective scar forms to allow the central nervous system to heal. It’s not unlike the body’s reaction to a run of the mill wound, but there’s a significant downside in this case.
While the resulting glial scar helps to stabilize the compromised area, it also walls it off—preventing the regrowth of nerve fibers that could enable renewed motor function like walking.
 William Murphy
William Murphy
A range of treatment options for spinal cord injuries are under development, but they’re predominantly focused on the acute phase, covering the days or weeks that follow when the glial scar still hasn’t fully formed. After that, patients run out of viable therapies.
A team of University of Wisconsin-Madison researchers, led by William Murphy, a professor of biomedical engineering, orthopedics and rehabilitation, is developing a novel method for breaking down the glial scar and paving the way for motor function recovery in patients with long-term spinal cord injuries.
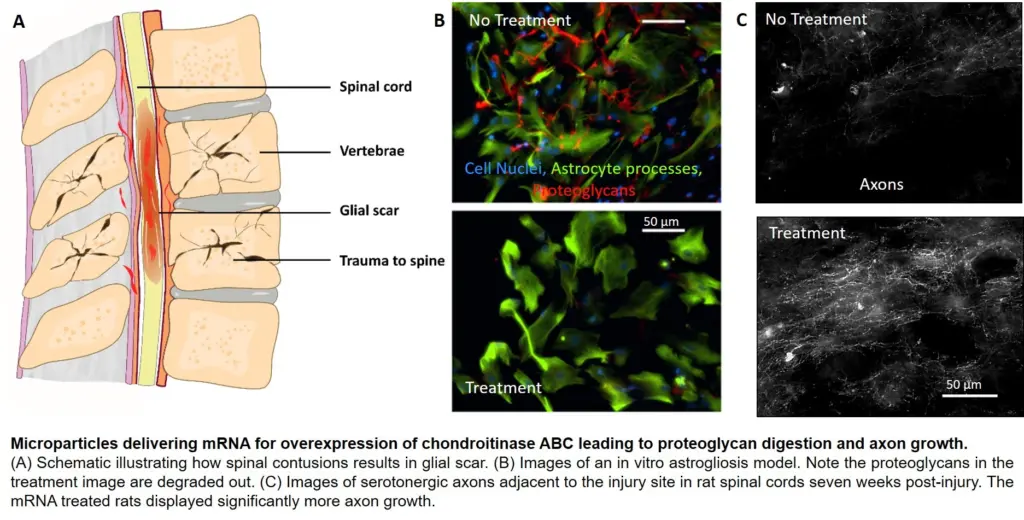
Murphy’s group, in collaboration with the lab of Amgad Hanna, a professor of neurological surgery in the UW-Madison School of Medicine and Public Health, is using mineral-coated microparticles to nonvirally deliver messenger RNA (mRNA) to direct production of an enzyme that can degrade the glial scar and potentially also drive new axon growth to restore movement. The team detailed its successful results in rat models in the journal Advanced Healthcare Materials. Alumnus Andrew Khalil (PhDBME ’17) was the paper’s first author.
“My hope is this opens up an opportunity to treat patients who have chronic spinal cord injuries,” says Murphy, whose lab develops biologically inspired materials for regenerative medicine applications. “That’s a patient population that really doesn’t have options right now.”
Through an injection directly into the glial scar, the microparticles carry mRNA that causes cells in the area to “overexpress” an enzyme called Chondroitinase ABC (ChABC), which can break down a key component of the glial scar.
Previous studies have demonstrated ChABC’s effectiveness in doing so. But the enzyme is unfortunately rather unstable, meaning its activity—its ability to catalyze a chemical reaction—drops rapidly. Synthetically produced ChABC is also less active than the version created in the body, and when injected, it’s transported away from the site.
The UW-Madison team’s solution is the first to use mRNA delivery to encode ChABC production, which results in a natural, more active version of the enzyme that’s released slowly over time and remains at the site of the scar.
“It’s a one-delivery system, so you don’t need repeated injections,” says Dan Hellenbrand (MSBME ’10), a researcher in Hanna’s lab who sustained a spinal cord injury in 2003, leaving him paralyzed.
The adaptable microparticles could also serve as a platform to deliver mRNA for production in the body of other difficult-to-produce therapeutic proteins, such as nerve growth factors, needed for spinal cord injury treatment.
In the group’s work on rat models, the researchers were surprised to see some motor function recovery with ChABC delivery alone, though Murphy is quick to note that achieving similar results in larger animal models and, ultimately, humans will almost certainly require a complementary treatment.
Murphy’s lab has previously created mRNA strands that encode for production of neurotrophin-3, a molecule that enhances axon growth. He and Hanna are already pursuing grants to test a combination mRNA therapy with ChABC and neurotrophin-3 in rodent models, with a long-term aim of human clinical trials and commercialization.
It’s the sort of research that inspired Hellenbrand to earn his master’s degree under the direction of Murphy and Hanna—and with the help and support of his wife, Amy—and continue working in the latter’s lab.
“My personal experience with spinal cord injury is the whole reason why I’m researching spinal cord injury and looking for treatments, not just for me, but for future generations,” he says.
Other UW-Madison-affiliated authors include: Biomedical engineering and MD-PhD student Joshua Choe, Kaitlyn Reichl (BSBME ’17), Jennifer Umhoefer (BS Integrative Biology ’17), Mallory Filipp (BS Biochemistry ’18).
Funding for this research came from National Institutes of Health grants R01AR059916, R21EB019558, R01NS109427, U01TR002383; National Science Foundation grant DMR 1105591; National Institute of General Medical Sciences’ Biotechnology Training Program grant NIH 5 T32 GM008349; National Science Foundation grant DGE-1256259; and National Institutes of Health grants TL1TR002375 and T32GM008692.
Top image caption: A microparticle shown via scanning electron microscopy. Image courtesy Andrew Khalil (PhDBME ’17).