For an illness like cancer, doctors often turn to computed tomography (CT) scans for a more definitive diagnosis, based on reconstructing a 3D organ from multiple 2D image slices. At the molecular level, such 3D scans could become an important part of precision medicine: a future of tailoring treatment decisions to each patient’s unique cellular features.
But translating the idea of CT scans from large organs, such as our heart or brain, to miniscule molecules is far from trivial—which is why Paul Campagnola, a professor of biomedical engineering and medical physics at the University of Wisconsin-Madison, has made a career out of it.
With a paper published this month (October 2017) in the journal Optica, he has now taken a crucial next step toward the 3D molecular imaging of collagen, the most abundant protein in humans that is found in all of our bones, tendons and connective tissues.
“Collagen is essential for bone and tissue stability, and changes in its intrinsic 3D organization are a key feature of all cancers and several other diseases,” Campagnola says. “That’s why detailed images of these changes could become an important part of clinical treatment decisions in the future.”
What makes collagen imaging so tricky? A traditional optical microscope depicts differences, or contrasts, between lighter and darker objects because they absorb different wavelengths of the light that shines through them. But since collagen molecules are transparent, they don’t generate those contrasts.
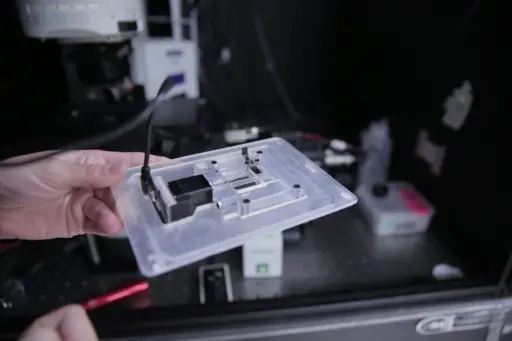 A 3D-printed custom device holds a motor (left) that spins the tube containing the sample. The device sits on the stage of an upright microscope while a laser source below the stage creates 2D images of the collagen contained in the rotating sample. Photo by Renee Meiller.
A 3D-printed custom device holds a motor (left) that spins the tube containing the sample. The device sits on the stage of an upright microscope while a laser source below the stage creates 2D images of the collagen contained in the rotating sample. Photo by Renee Meiller.
Special techniques are available to image transparent objects, but in the case of collagen, Campagnola and other researchers demonstrated in the late 1990s that higher-resolution 2D images result from exploiting its rigid and hierarchical structure: Individual collagen molecules are stacked together like a brick wall into collagen fibrils, which are packed side-by-side into parallel bundles called collagen fibers. It is this structure that gives collagen-based body parts their almost steel-like stability.
And while such a highly organized transparent structure does not change light’s primary frequency, it interacts with its so-called “second harmonic” frequency. In music, the second harmonic of a sound wave has twice the frequency and half the wavelength of the original, creating a sound one octave higher on a string instrument.
“Collagen is the most common human tissue type whose interaction with a laser creates a new unique signal that we call second harmonic light, analogous to music’s second harmonic sound,” Campagnola explains. “Unlike other materials, collagen’s molecules assemble in such a way that this light is bright and can distinguish between different substructures.”
Thus, second harmonic generation microscopy was born when researchers learned how to convert these higher-order signals into 2D images—but 3D images remained elusive for a few more years.
With their new study, Campagnola’s group has now provided the experimental and computational framework for assembling 2D collagen images, taken from multiple angles around the tissue sample, into a moderate-resolution 3D view, similar to the familiar CT scan of human organs.
Key to this new imaging paradigm is a 3D-printed device that holds a tube attached to a small motor and sits on the stage of an upright microscope. Once a tissue sample (say, a mouse tail tendon) is placed into the tube, the motor starts to spin it. Every time a laser source, located below the stage, sends light through the rotating sample, a laser scanner records the resulting 2D microscope image. At the end of the procedure, a complex mathematical algorithm reconstructs a 3D image—a first step toward second harmonic generation tomography—from all of the 2D slices.
Once deployed in clinical settings, high-resolution 3D collagen tomography may hone in, for example, on subtle differences between highly aligned collagen fibers in breast and ovarian cancer tissue, which are distinct from the cross-hatched mesh of collagen found in normal tissue. These images may inform treatment decisions not only for cancer, but also for pulmonary fibrosis, a condition in which damaged and scarred lung tissue reduces a patient’s ability to breathe.
“Our next goal is to apply the new technology to a variety of diseased tissues,” Campagnola says. “If we can build a large enough patient database with both images and clinical outcomes, physicians can eventually choose chemotherapy or other treatments based on the 3D collagen structure in a patient’s own tissue—which is the kind of precision medicine that can really make a difference in treatment success.”
The study was a collaboration with the Morgridge Institute for Research, whose 3D printing facility is directed by co-author Kevin Eliceiri. First author Kirby Campbell recently completed his PhD in biomedical engineering and is now a postdoctoral fellow at the St. Jude Children’s Research Hospital in Tennessee. The study was funded by the National Science Foundation (CBET-1402757) and the National Institutes of Health (5T32CA9206-38, 1R01CA206561-01, and R21 HL126190).