To record brain activity, neuroscientists and physicians must choose between electrodes that require wires running through the skull or noninvasive options like electroencephalography (EEG) or magnetoencephalography (MEG) that track signals from the scalp.
In addition to electrodes’ potential to cause injury when implanted, they’re limited by where they can spatially reach in the brain. EEG and MEG, unsurprisingly, are prone to weak signals and, like electrodes, collect data from populations of neurons in specific locations. MRI, another option for scanning the brain, merely tracks blood flow as an indirect indicator of brain activity.
In short, each offers a limited picture of the brain, leaving researchers to explore ways to get a more comprehensive look at neural activity. That’s why Biomedical Engineering Assistant Professor Aviad Hai’s lab at the University of Wisconsin-Madison is developing technologies to deliver a wider, deeper and more granular view, including a unique nanoscale device capable of forming direct connections with individual brain cells and amplifying their magnetic signals.
 Jack Phillips
Jack Phillips
“It’s an entirely different framework for thinking about brain recording than the current methodologies that are electrode-based,” says Jack Phillips, a PhD student in Hai’s lab.
Phillips, Hai and other lab members detailed and modeled the capabilities of one such untethered neural sensor in a July 2022 paper in the Journal of Neural Engineering, laying the groundwork for systems capable of detecting signals at the single-cell level across different regions of the brain.
“We miniaturized the interface between brain cells and wireless nano antennas, to the point where now we have antennas that are the size of cells,” says Hai. “So we basically have a one-to-one relationship between brain cells and devices that transmit brain activity to the outside.”
Their “nano antenna”—made of gold, because of its electrical conductivity and biocompatibility—includes a mushroom-shaped interface pad with a geometry that encourages a connection with a neuron resembling the synapse it naturally forms with other neurons. Another pad acts as a ground, creating a voltage difference that drives electrical current through a coil, which in turn amplifies the neuron’s intrinsic magnetic signal.
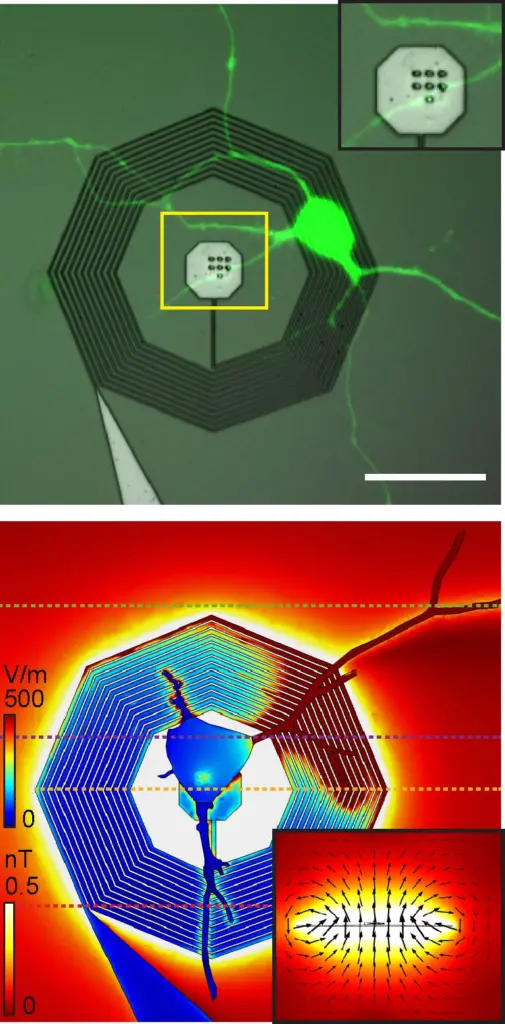 Top: A fluorescently labeled neuron growing on top of a nanofabricated coil. Bottom: A model of the strength of the electrical and magnetic fields from the neuron when connected to the nanofabricated coil.
Top: A fluorescently labeled neuron growing on top of a nanofabricated coil. Bottom: A model of the strength of the electrical and magnetic fields from the neuron when connected to the nanofabricated coil.
In the paper, Phillips created a 3D model of the device in the software platform COMSOL Multiphysics, using a mathematical model developed by undergraduate researcher Mitchell Glodowski (BSBME ’20) to optimize its design. Then, using baseline electric activity measured in cultured neurons, Phillips modeled the enhanced magnetic signal strength. They found that signal was more than 250 times stronger than the neuron’s intrinsic signal.
Hai and several lab members are currently working on another paper outlining the nanoscale lithography techniques they’re using to fabricate the devices, and the group plans to validate the antennas in cell cultures and animal models.
While it’s a long way from modeling sensors on a computer to injecting them into human patients, Hai believes his group is building the basis for a system that could provide broader, denser and deeper brain monitoring to more comprehensively track abnormalities, power brain-machine interfaces and more.
“You can’t record from one area in the brain and expect to understand how it works,” he says. “There are so many regions in the brain that participate in just about every task that we need to find standalone devices that can be deployed across the brain without injuring it or occupying so much real estate.”
Assistant Professor Aviad Hai is also part of the Grainger Institute for Engineering’s neuroengineering focus and the Wisconsin Institute for Translational Neuroengineering, both at UW-Madison. In addition to Hai, PhD student Jack Phillips and recent alumnus Mitchell Glodowski (BSBME ’20), other authors on the paper include graduate student Yash Gokhale, alumnus Matthew Dwyer (MSEE ’11, PhDEE ’17) and staff scientist Alireza Ashtiani (MSEE ’12, PhDEE ’16).
Funding for the research came from the National Institute of Neurological Disorders and Stroke and the Office of the Director’s Common Fund at the National Institutes of Health via Grant DP2NS122605, the National Institute of Biomedical Imaging and Bioengineering via Grant K01EB027184 and the Wisconsin Alumni Research Foundation.
Top photo caption: The research team modeled the device’s magnetic response, shown in the plane and magnetic flux lines, to a neuron’s electrical signals, shown on the neuron and coil.