Although many Americans dutifully deposit their plastic trash into the appropriate bins each week, much of that material, including flexible films, multilayer materials and a lot of colored plastics are not recyclable using conventional mechanical recycling methods. In the end, only about 9% of plastic in the U.S. is ever reused. Equally discouraging, the products made from recycled plastic typically are low-value, meaning the high costs of recycling don’t necessarily yield great returns.
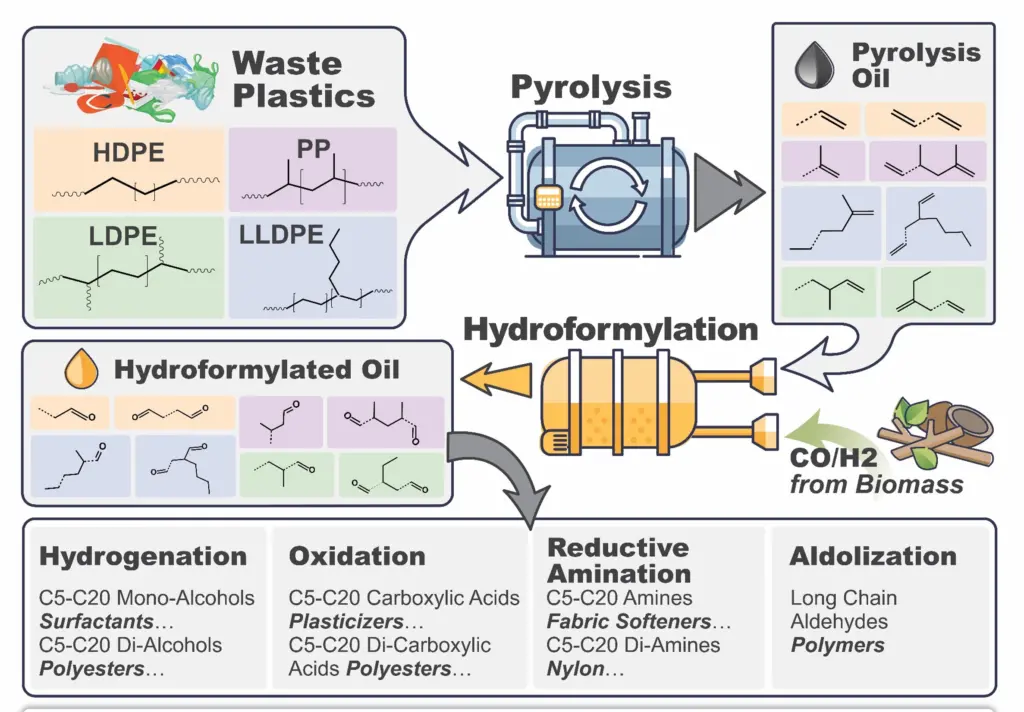
With a new technique, however, University of Wisconsin-Madison chemical engineers can turn waste plastic into high-value chemicals. The method relies on two chemical processes—pyrolysis and hydroformylation—which ultimately increase the economic incentives for plastic recycling and open the door to recycling new types of plastic.
 Heating plastic waste, like this, in a low-oxygen environment creates an oil that can further processed into high-value chemicals. Credit: Joel Hallberg.
Heating plastic waste, like this, in a low-oxygen environment creates an oil that can further processed into high-value chemicals. Credit: Joel Hallberg.
The team estimates its methods could reduce greenhouse gas emissions from the conventional production of these industrial chemicals by roughly 60 percent.
The researchers, led by George W. Huber, a professor of chemical and biological engineering at UW-Madison and director of the Department of Energy-funded Center for the Chemical Upcycling of Waste Plastics, postdoctoral researcher Dr. Houqian Li and PhD student Jiayang Wu, described their methods in the August 11, 2023 issue of the journal Science.
The world is swimming in plastic waste—in some cases literally—with few options for recycling much of that material. One emerging method is pyrolysis, in which plastics are heated to high temperatures in an oxygen-free environment. The result is pyrolysis oil, a liquid mix of various compounds. Pyrolysis oil contains large amounts of olefins—a class of simple hydrocarbons that are the central building blocks of today’s chemicals and polymers, including various types of polyesters, surfactants, alcohols and carboxylic acids.
In current energy-intensive processes like steam cracking, chemical manufacturers produce olefins by subjecting petroleum to extreme high heat and pressure. In their new process, the UW-Madison team recovers olefins in the pyrolysis oil and then uses them in a much less energy-intensive chemical process called homogenous hydroformylation catalysis. The process converts olefins into aldehydes, which can then be further reduced into important industrial alcohols.
“These products can be used to make a wide range of materials that are higher-value,” says Huber. “You can make surfactants from them that are used to make soaps and cleaners. You could make other polymers from them that are of higher value than the original polyolefins. So, we’re really excited about the implications of this technology. It’s a platform technology to upgrade plastic waste using hydroformylation chemistry.”
The recycling industry could adopt the process soon; in recent years, at least 10 large chemical companies have built or announced plans for facilities to produce pyrolysis oils from waste plastics. Many of them run the pyrolysis oil through steam crackers to produce low-value compounds. The new chemical recycling technique could provide a more sustainable and lucrative way to use those oils. “Currently, these companies don’t have a really good approach to upgrade the pyrolysis oil,” says Li. “In this case, we can get high-value alcohols worth $1,200 to $6,000 per ton from waste plastics, which are only worth about $100 per ton. In addition, this process uses existing technology and techniques. It’s relatively easy to scale up.”
 Credit: Xin Zou.
Credit: Xin Zou.
The collaborative nature of UW-Madison and the Department of Chemical and Biological Engineering made this project a team effort, Huber says. Clark R. Landis, chair of the Department of Chemistry and a world expert on hydroformylation, suggested the possibility of applying the technique to pyrolysis oils. Chemical and Biological Engineering Professor Manos Mavrikakis used density functional theory to provide molecular-level insight into how the chemical bonds break. And Chemical and Biological Engineering Professor Victor Zavala provided help analyzing the economics of the technique and the life cycle of the plastic waste.
The next step for the team is to tune the process and better understand what recycled plastics and catalyst combinations produce which final chemical products. “There are so many different products and so many routes we can pursue with this platform technology,” says Huber. “There’s a huge market for the products we’re making. I think it really could change the plastic recycling industry.”
George Huber is the Richard L. Antoine Professor; Manos Mavrikakis is the Ernest Micek Distinguished Chair, James A. Dumesic Professor, and Vilas Distinguished Achievement Professor; and Victor Zavala is the Baldovin-DaPra Professor.
Other UW-Madison authors include Zhen Jiang and Jiaze Ma.
The authors acknowledge support from the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Bioenergy Technologies Office under Award Number DEEE0009285; The National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the DOE, Office of Science, under contract no. DE-AC02-05CH11231 using NERSC award BES-ERCAP0022773; The Center for Nanoscale Materials, a DOE Office of Science User Facility located at Argonne National Laboratory supported by DOE contract DE-AC02-06CH11357; and the UW-Madison Center for High Throughput Computing supported by UW-Madison, the Advanced Computing Initiative, the Wisconsin Alumni Research Foundation, the Wisconsin Institutes for Discovery, and the National Science Foundation.
Featured image caption: Using a process called hydroformylation, Professor George Huber (left) and postdoctoral researcher Houqian Li are able to recover the olefins in an oil made from waste plastic and transform them into high-value chemicals. Credit: Joel Hallberg.